Introduction: Young adults (YAs), ages 18-39 years diagnosed with cancer have distinct physical, developmental, and psychosocial needs that are often unmet during oncologic treatment. In acute myeloid leukemia (AML), a disease affecting both younger and older adults, patients navigate several treatment intensity choices, and with intensive induction chemotherapy are often balancing a chance of cure with a risk of death. Furthermore, YAs with AML can have marked life disruptions with this unpredictable illness trajectory and prolonged induction hospitalization, further conflicting with normal development at this stage. Fundamentally different than solid tumors, we lack an understanding of the YA experience in AML induction compared to their older counterparts. This study explores patient-reported outcomes (PROs) including quality of life (QOL) and physical and psychological symptom burden among YAs undergoing induction chemotherapy.
Methods: We conducted a secondary analysis of 160 patients with high-risk AML hospitalized to receive intensive chemotherapy who were enrolled in a randomized control trial of palliative care (PC) intervention versus standard oncology care. Patients with newly diagnosed, relapsed, or refractory AML receiving intensive treatment requiring a 4-6 week hospitalization were enrolled in the study. We used the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu), the Edmonton Symptom Assessment System (ESAS), the Hospital Anxiety and Depression Scale (HADS), and the Post-Traumatic Stress Disorder (PTSD) Checklist to assess QOL, symptom burden, and psychological distress respectively. Assessments were administered at baseline, week-2, and week-4 following initiation of chemotherapy. We used descriptive statistics to compare PROs at baseline between YAs (< 40 years) and older adults. We used linear regression models controlling for PC intervention effect when examining the association between age (YAs versus older adults) and PROs at week-2. We used linear mixed effect models controlling for randomization to the PC intervention to assess the association between age and PROs longitudinally throughout the hospitalization.
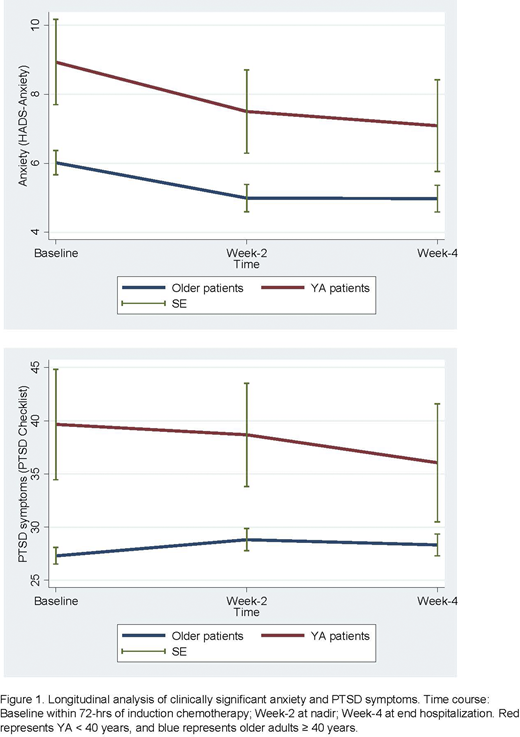
Results: Of the 160 patients with high-risk AML enrolled in this study, 14 (8.8%) were YAs < 40 years. Demographic characteristics highlight that the majority of YAs had relapsed AML (n=10, 71.4%) and there was a greater presence of racial and ethnic diversity compared to older adults. The baseline mean QOL score for YAs was 110.5 (SD 34.2). Overall, 57.1% (8/14) reported clinically significant anxiety and 50% (7/14) reported clinically significant PTSD symptoms at baseline. Fewer YA patients, 21.4% (3/14) reported clinically significant depression. Evaluation of PROs at week-2 nadir adjusted for randomization to the PC intervention, highlighted a significant lower QOL (β=-18.27; p=0.036), higher anxiety (β=2.72; p=0.048), and higher PTSD symptoms (β=10.34; p=0.007) for YAs compared to older adults. Longitudinal analyses exploring the impact of age on PROs across induction hospitalization demonstrated that YAs had consistently significant higher anxiety (β=3.66; p=0.024) and PTSD symptoms (β=15.64; p<0.001) compared to older adults [Figure 1].
Conclusions: For YAs with AML undergoing induction chemotherapy, anxiety and PTSD symptoms are highly prevalent both at baseline and longitudinally throughout the induction hospitalization. This demonstrates a continued unmet need of the significant psychological distress and intensified hospitalization experience for YAs compared to their older counterparts. Given that distress during induction hospitalization can be predictive of post-treatment outcomes, it is imperative to tailor integrated supportive care and psychosocial interventions for YAs with AML during induction chemotherapy.
Walker:Geron: Research Funding; Newave Pharmaceuticals: Research Funding. Bhatnagar:KaryoPharm Therapuetics: Research Funding; Cell Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; KITE: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.